

MINIject® is iSTAR Medical’s innovative minimally-invasive glaucoma surgery (MIGS) device for patients with open-angle glaucoma.
MINIject® is currently the only commercially available MIGS implant targeting the supraciliary space, which is shown to deliver safe, meaningful and sustained control of intraocular pressure (IOP).

MINIject® is designed to significantly reduce IOP by enhancing natural outflow from the anterior chamber to the supraciliary space. Implantation is predictable in a single-step procedure using a deployment wheel.
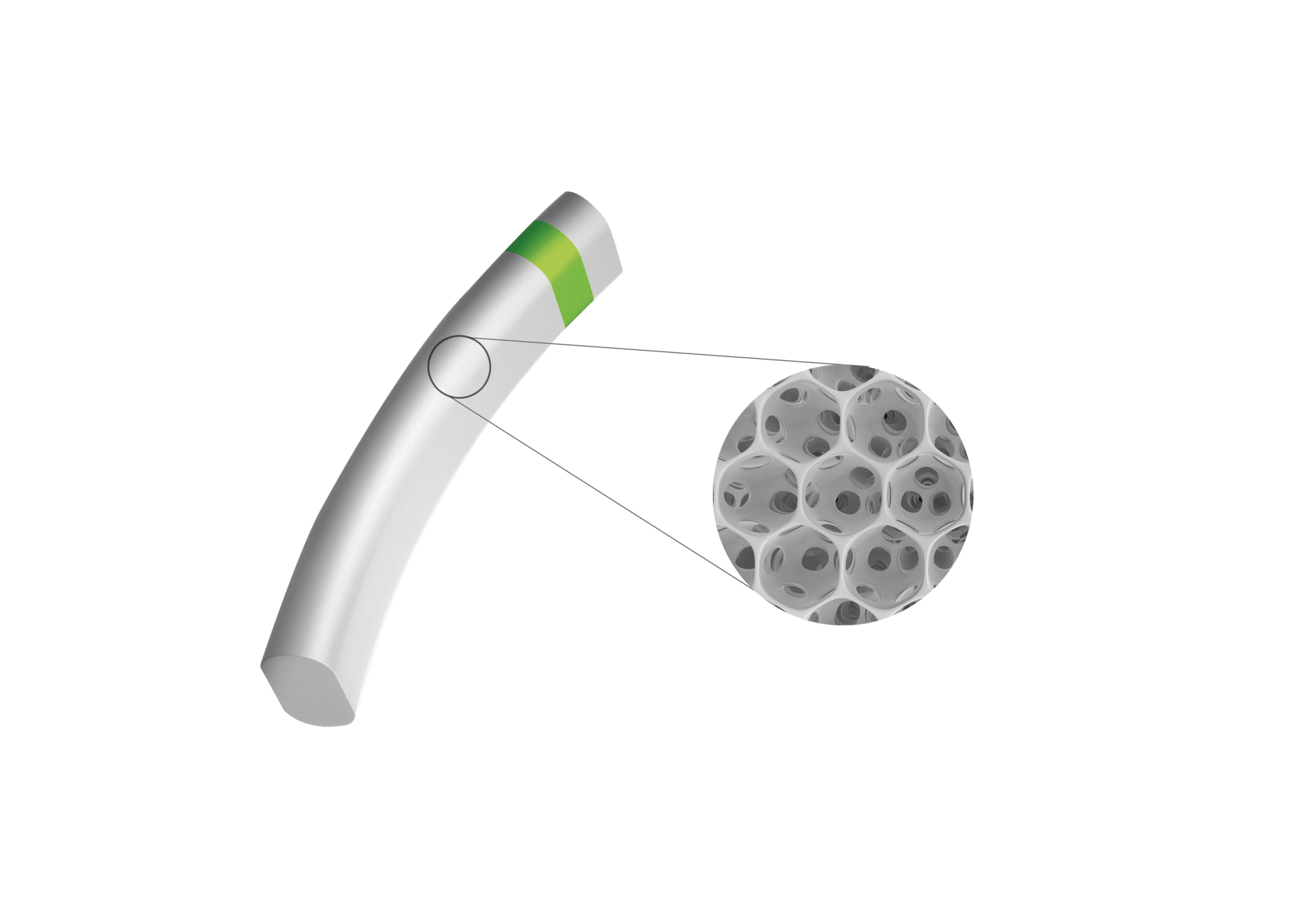
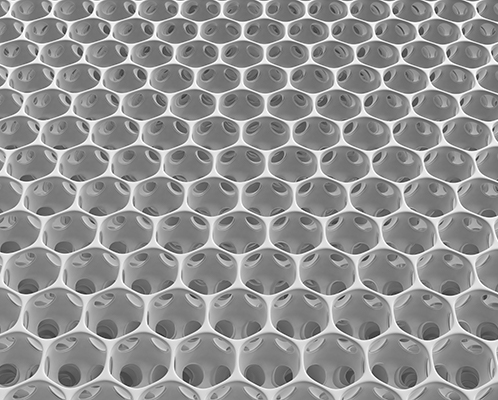
MINIject®’s distinctive, soft and flexible porous structure delivers multiple benefits:
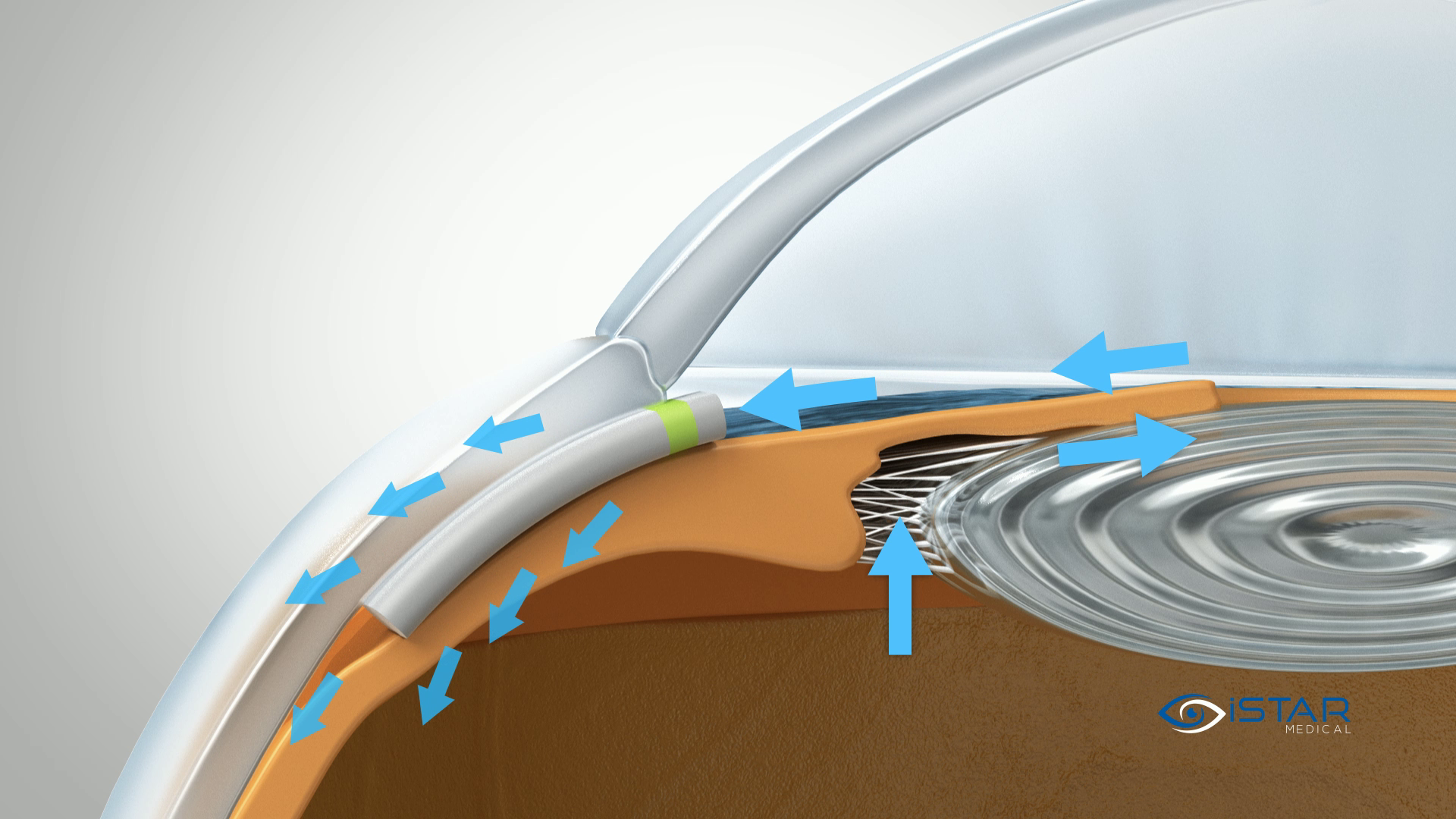
MINIject® is currently the only commercially available MIGS device that enhances natural flow in the supraciliary space with bio-integration, for safe, meaningful and sustained control of IOP.
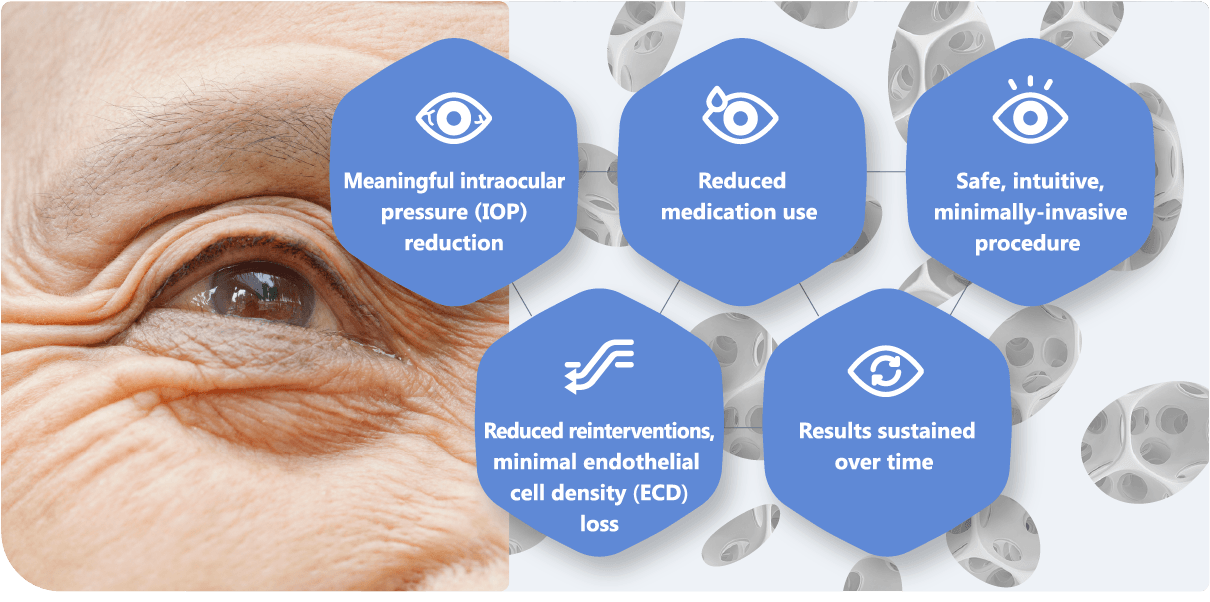

Clinical data of MINIject® in standalone trials at two-year follow-up has shown: 3
MINIject (FG1004)
Version 13, 2022-09-16
English / German / Spanish / French / Italian / Dutch
Version 13, 2022-09-16
Swedish / Norwegian / Finnish / Portuguese
MINIject (FG1004)
Version 12, 2022-08-18
English / German / Spanish / French / Italian / Dutch
Version 12, 2022-08-18
Swedish / Norwegian
Version 11, 2021-10-04
English / German / Spanish / French / Italian / Dutch
Version 10, 2021-07-14
English / German
PATIENT SAFETY INFORMATION
Please contact us at [email protected] if you require further information.
MINIject® is only available for sale in the European Union, the UK, Norway, Iceland, Switzerland and Australia.
MINIject® safety and efficacy results are currently sustained out to 3 years, as presented by Dr Ike Ahmed at WGC 2021.
1 Grierson I, Minckler D, Rippy MK et al. “A novel suprachoroidal microinvasive glaucoma implant: in vivo biocompatibility and biointegration.” BMC biomed eng 2, 10 (2020).
2 Ultrasound Biomicroscopy (UBM) images on file
3 Data on file and presented by CEO Michel Vanbrabant at Ophthalmology Innovation Summits (OIS) in 2021. Denis P, Hirneiß C, Durr GM, et al. “Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial”. Br J Ophthalmol 2020;0:1–6