

A comprehensive clinical trial program supports the development of MINIject®, with positive combined results at two years from the STAR-I and STAR-II clinical trials shown below. 1
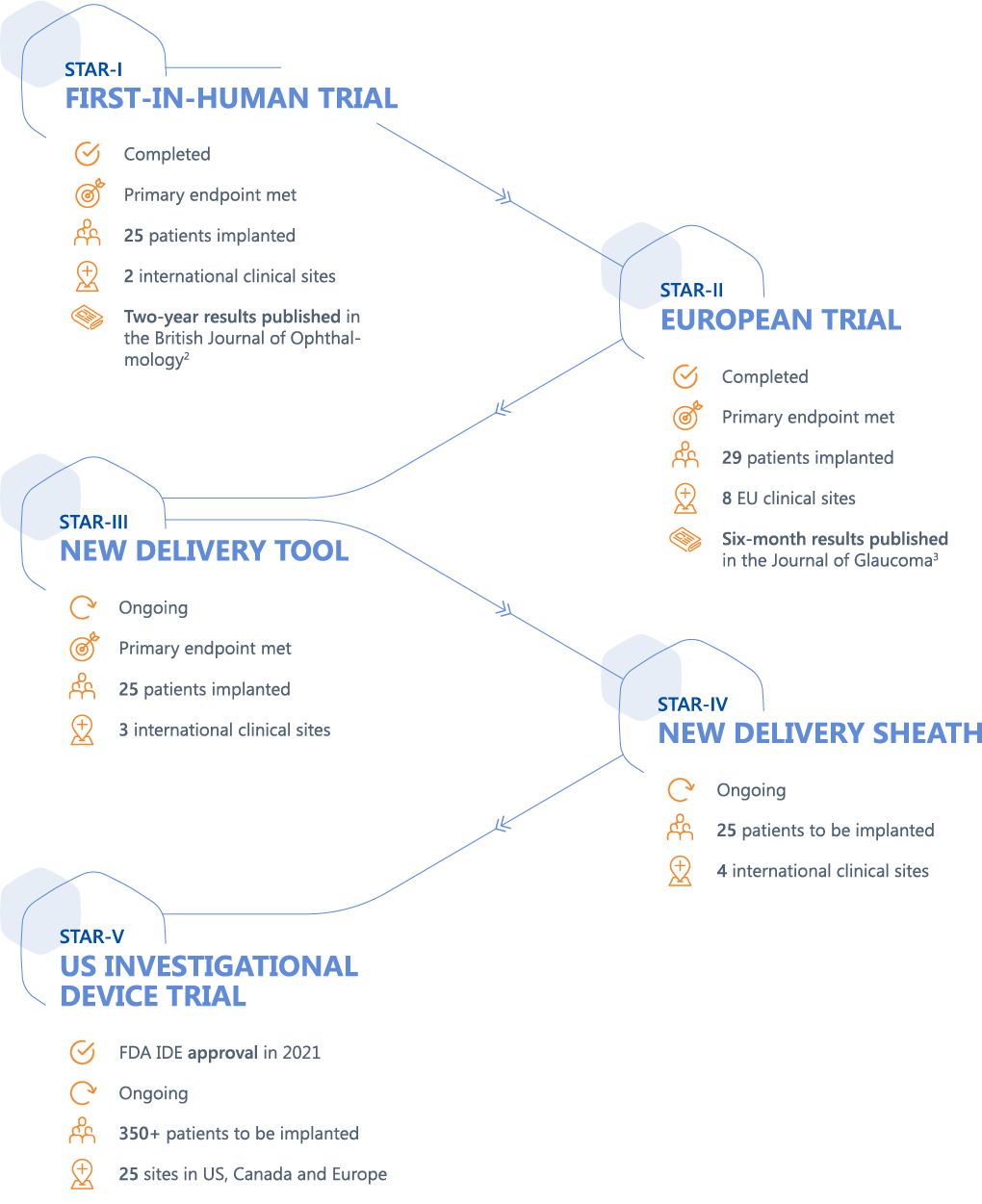
1 Data on file and presented by CEO Michel Vanbrabant at Ophthalmology Innovation Summits (OIS) in 2021.
2 Denis P, Hirneiß C, Durr GM, et al. “Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial”. Br J Ophthalmol 2020;0:1–6
3 García Feijoó J, Denis P, Hirneiß C et al. “A European Study of the Performance and Safety of MINIjectTM in Patients With Medically Uncontrolled Open-angle Glaucoma (STAR-II)”. J Glaucoma. 2020 Oct;29(10):864-871